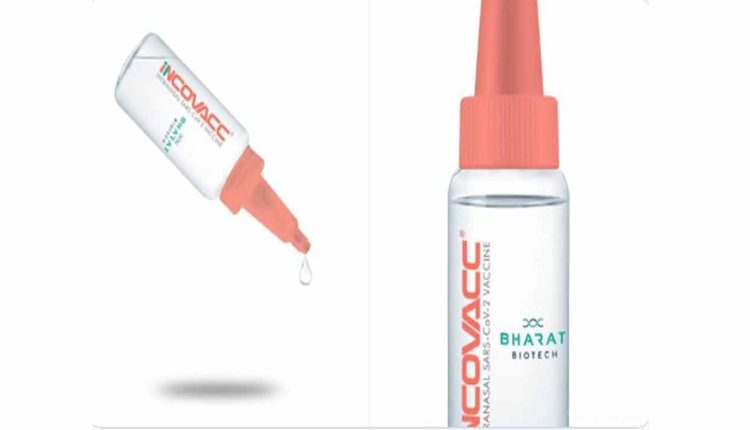
Bharat Biotech COVID Nasal Vaccine Gets CDSCO Approval
Hyderabad, Nov 28 (Maxim News): The Hyderabad based global vaccine maker Bharat Biotech International Limited announced that its INCOVAC (BBV154), its COVID-19 vaccine in the form of nasal drops, has received approval from the Central Drugs Standard Control Organisation (CDSC) under restricted use in emergency situation for ages 18 and above, in India as booster doses.
This vaccine was evaluated in phases I, II and III clinical trials with successful results. The nasal delivery system has been designed and developed to be cost effective in low-and-middle-income countries. INCOVACC was developed in partnership with Washington University. St. Louis, which had designed and developed the recombinant adenoviral vectored construct and evaluated in preclinical studies for efficacy. (Maxim News)
Next Story :
Now you can get latest stories from Indtoday on Telegram everyday. Click the link to subscribe. Click to follow Indtoday Facebook page and Twitter and on Instagram. For all the latest Hyderabad News updates